MIT Researchers Create Cheap 'Air-Breathing' Battery For Green Energy
Scientists from the Massachusetts Institute of Technology have spent years developing a cheaper large-scale battery for electrical grids powered by renewable energy. They have finally created a battery that uses sulfur, liquid salt, and oxygen.
Updated May 25 2019, 1:10 a.m. ET
In order to make renewable energy reliable on a large scale, there needs to be a way to store the power that's being generated. Batteries are the best solution, but lithium-ion can be a costly product and they lose capacity after every recharge. Researchers at the Massachusetts Institute of Technology have developed a more natural battery that’s not only reliable, but much less expensive.
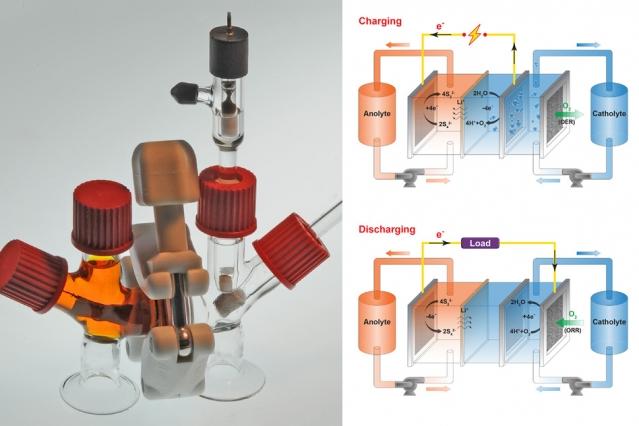
This new battery is labeled as “air-breathing,” meaning that oxygen does push in and out of it to make it work. Sulfur dissolved in water is the anode (negative side) of the battery while a liquid salt solution represents the cathode (plus side). When oxygen moves into the battery, electrons are released from the anode.
How does the battery get recharged? Oxygen moves out of it so the electrons come back into the anode. Yet-Ming Chiang, one of the researchers and a co-author of the project’s report, explained that the battery breathes in and exhales oxygen -- it’s not like humans that release carbon dioxide.
The project began back in 2012 with the focus on creating a large-scale battery that can be used to store energy for the grid. Chiang told MIT News that he didn’t believe the high cost of lithium-ion would be sustainable in the long run: “This meant maybe we weren’t focusing on the right thing, with an ever-increasing chemical cost in pursuit of high energy-density. We said, ‘If we want energy storage at the terawatt scale, we have to use truly abundant materials.’”
During the research process, sulfur was immediately decided as the battery’s anode. However, it took a while for the scientists to find a compatible liquid cathode that was cheap. Prior to using liquid salt, one of the chemical compounds that tested was potassium permanganate. While it didn’t end up working, they found the battery ended up recharging due to its reaction from the oxygen.
With that discovery, they ended up creating an ultra-cheap battery. According to the news article, the combination of all three parts of this battery would be “1/30th the cost of competing batteries, such as lithium-ion batteries.” It’s one of the cheapest rechargeable batteries that you could make.
Even though the prototype is very small -- the scientists say it’s no bigger than a coffee cup -- the battery can easily be scaled up to store energy in the grid. Power can be stored for seasons at a time, but it can discharge easier than other alternatives. For this reason, storing electricity from wind and solar-generated power might be the best use for it.
This isn’t the first time salt has been used in a renewable energy storage process. Google has been researching a process that uses salt and antifreeze and is also fairly cheap. Solar and wind power may not be reliable on its own, but we’re finding amazing ways to store this energy so we don’t have to keep using fossil fuels.