FDA Warns That Counterfeit Ozempic Has Flooded U.S. Drug Markets — Here's What to Know
Consumers should immediately report adverse side effects to the FDA.
Published April 15 2025, 2:11 p.m. ET
Among patients for whom drugs like Ozempic are prescribed, the health benefits may be profound. However, for the countless patients who are instead seeking compounded Semaglutide from various pharmacies, the adverse side effects, as well as the lack of intended results, can be immensely frustrating — and dangerous.
While speculation is rampant that Ozempic carries potential short- and long-term risks, you can imagine that counterfeit Semaglutide carries a litany of unknown risks.
Whether you or your family and friends have been prescribed Semaglutide for certain health conditions, you should be aware that counterfeit versions of the drug have once again entered the U.S. drug market for the umpteenth time, posing risks to patients everywhere.
Here's what we know so far about the April 14 U.S. Food and Drug Administration (FDA) announcement and what patients should do if they discover their Semaglutide is illegitimate.
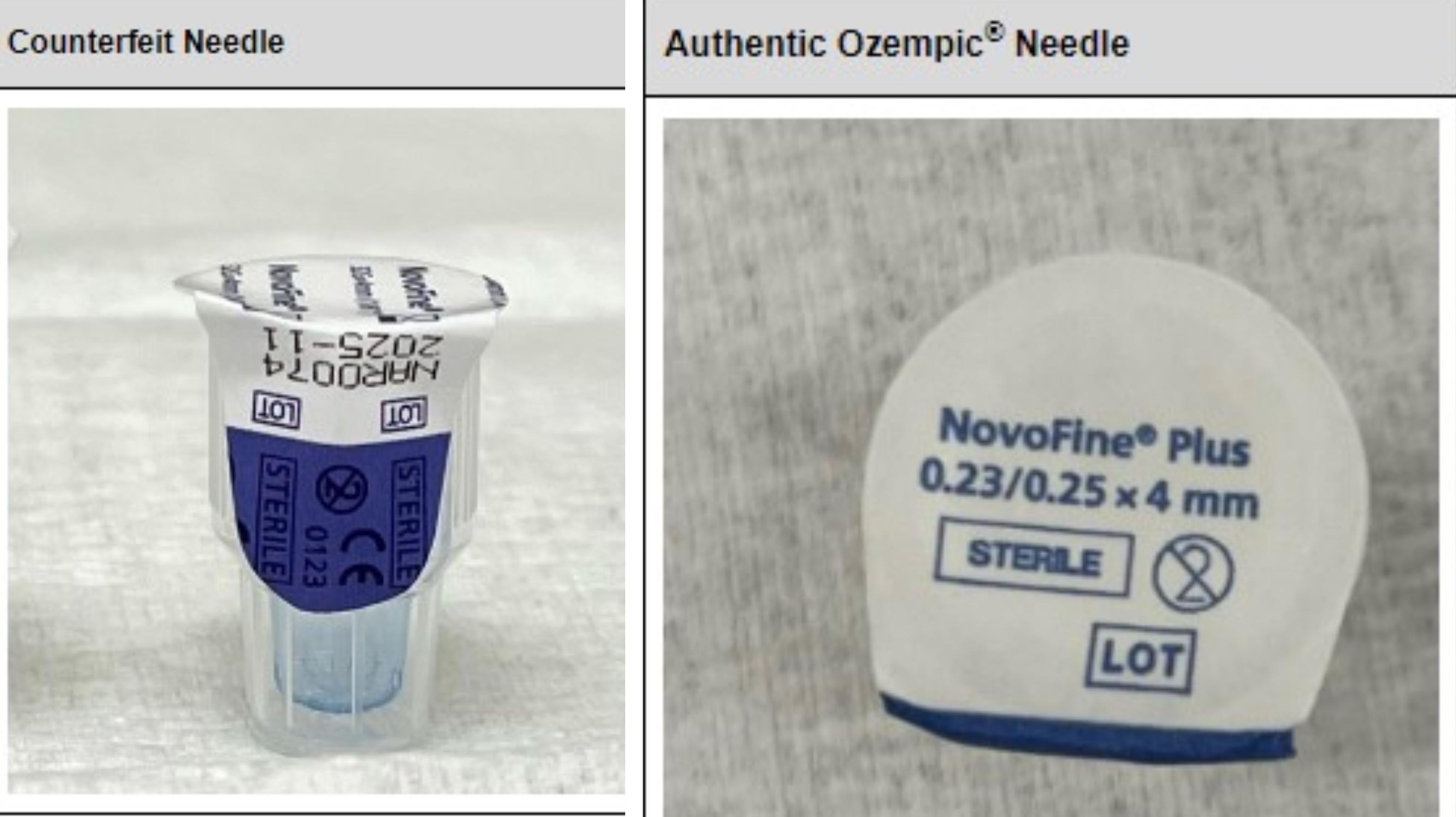
Do you have counterfeit Ozempic? Check for this lot number.
On April 14, Novo Nordisk, the manufacturer of Ozempic, disseminated a press release in conjunction with the FDA to notify healthcare providers and patients that the pharmaceutical giant seized unauthorized supplies of 1 mg Semaglutide in the U.S.
The unauthorized distribution of counterfeit Semaglutide deceptively contains lot number PAR0362, which is also included in authentic Ozempic packages. It is tricky, to say the least, for unknowing patients to decipher the difference.
According to Novo Nordisk, the counterfeit versions of Semaglutide contain an illegitimate serial number.
If the first eight digits of the package's serial number begin with 51746517, providers and patients should consider the medication unauthorized and counterfeit.
Thankfully, the FDA notes that Novo Nordisk's reports of patients experiencing adverse side effects have not yet occurred from the counterfeit products.
"FDA is aware of six adverse event reports associated with this lot, however, none of them appear to be associated with the counterfeit product. All six adverse events were reported by Novo Nordisk," according to the FDA's press release.
Retail pharmacies, per Novo Nordisk, are advised to contact customer service to assess the legitimacy of their Ozempic supply. Pharmacies and medication providers are encouraged to only purchase Ozempic from the manufacturer's list of authorized distributors.
Patients, too, are encouraged to check the lot number and serial number on their packages to determine whether or not their supply is legitimate.
Counterfeit Ozempic is an ongoing problem in the U.S.
As the FDA's press release indicates, this is not the first time the agency has warned healthcare providers and patients of counterfeit Ozempic. In 2023, the FDA also seized thousands of units of counterfeit Ozempic products after the illegitimate products were distributed by pharmacies and other avenues not authorized by Novo Nordisk.
Sadly, as the above X post from toxicologist Josh Trebach, MD also indicates, the problem has remained persistent since Ozempic hit the market.
If you are experiencing adverse side effects from Ozempic or the counterfeit product, the FDA recommends submitting a report to the FDA’s MedWatch Safety Information and Adverse Event Reporting Program.